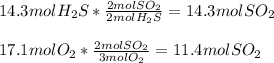Chemistry, 09.06.2021 07:50 Miloflippin7339
How much energy is used when 14.3 moles of hydrosulfuric acid reacts with 17.1 moles
of oxygen?
2 H2S + 3 02 + 175 KJ
—->2 SO2 + 2 H20
Which substance is the limiting reactant?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, angeljohnson2081
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1


Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 22:30, lanashanabJHsbd1099
Who discovered a pattern to the elements in 1869?
Answers: 1
You know the right answer?
How much energy is used when 14.3 moles of hydrosulfuric acid reacts with 17.1 moles
of oxygen?
Questions in other subjects:




Chemistry, 07.07.2019 01:30

History, 07.07.2019 01:30


Health, 07.07.2019 01:30


Health, 07.07.2019 01:30