
Chemistry, 09.06.2021 03:10 RealSavage4Life
Please help! Due tonight! Determine the reaction rate, with respect to nitrogen, for the reaction: N2(g) + 3H2(g) → 2NH3(g), between 10s and 50s, based on the following table:
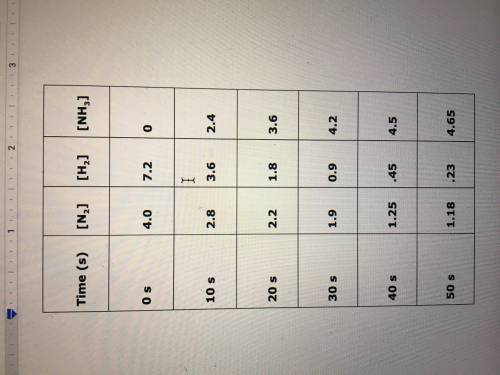

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2
You know the right answer?
Please help! Due tonight!
Determine the reaction rate, with respect to nitrogen, for the reaction:...
Questions in other subjects:












