Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
You know the right answer?
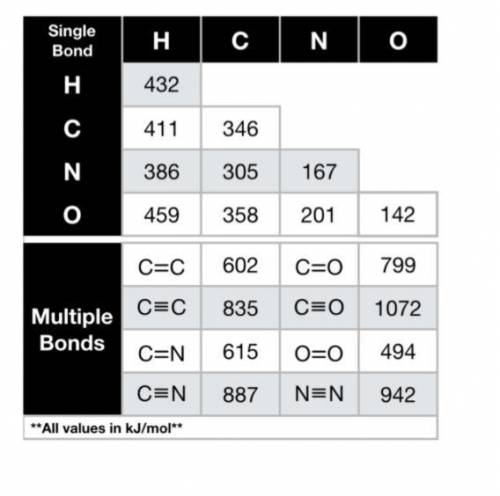
Using the bond energies provided, calculate the enthalpy of the reaction (∆Hrxn, in kJ) for the comb...
Questions in other subjects:


History, 29.07.2021 20:30


Mathematics, 29.07.2021 20:30

Chemistry, 29.07.2021 20:30


Mathematics, 29.07.2021 20:30


English, 29.07.2021 20:30

Mathematics, 29.07.2021 20:30