
Chemistry, 08.06.2021 08:50 elizabethprasad2
Assume that the reactions shown below are each an elementary step. What is molecularity for each reaction? What is rate law for each reaction?
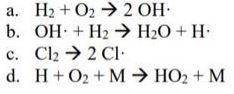

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 10:30, Brookwiggington8814
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1


Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2
You know the right answer?
Assume that the reactions shown below are each an elementary step. What is molecularity for each rea...
Questions in other subjects:

Mathematics, 10.04.2020 20:23

English, 10.04.2020 20:23

Mathematics, 10.04.2020 20:23



Mathematics, 10.04.2020 20:23




Mathematics, 10.04.2020 20:23



