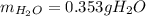Chemistry, 06.06.2021 01:30 alannaswitzer
Calculate the mass of water vapor that is produced by the reaction: 
1.4 g of CO2 and 2.2 g of KOH in the reaction: CO2 + 2KOH → K2CO3 + H20
Please show work, will give brainliest

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 10:00, valdezlizbeth6652
Why is carbon ideal for making different compounds?
Answers: 2


You know the right answer?
Calculate the mass of water vapor that is produced by the reaction: 
1.4 g of CO2 and 2.2 g of KOH...
Questions in other subjects:

History, 21.04.2021 19:40

English, 21.04.2021 19:40

Biology, 21.04.2021 19:40

Spanish, 21.04.2021 19:40

History, 21.04.2021 19:40

Social Studies, 21.04.2021 19:40


English, 21.04.2021 19:40

Biology, 21.04.2021 19:40