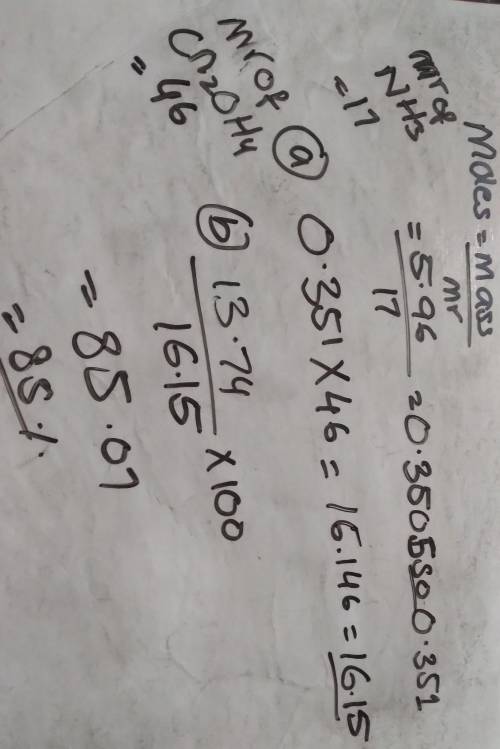Chemistry, 05.06.2021 23:00 deaishaajennings123
5.96 g of ammonia reacts completely according to the following reaction:
2 NH3, (g) + Co2, (g) → CN2,OH4, (s) + H20 (l)
(a) What is the theoretical yield of urea (CN, OH,) for this reaction?
(b) If 13.74 g of urea are produced, what is the percent yield for this equation?
please show work, will give brainliest

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:30, neariah24
Plz me ! 1 which of earths spheres contains most of its mass? a atmosphere b hydrosphere c geosphere* d biosphere 2 erosion and weathering are examples of which types of forces? a constructive forces b destructive forces* c gravitational forces d inertia-related forces 3 which of the following statements about earths atmosphere is true? a earths atmosphere contains 78% water vapor which is essentail to life b earths atmosphere contains 21% oxygen c earths atmosphere contains carbon dioxide which all life forms require d earths atmosphere allows radiation from the sun to pass through it and warm earths surface* 4 the strenght of the force of gravity between two objects is determined by which of the following factors? select all that apply a the messes of the objects* b the distance between the objects* c the volumes of the objects d the surface area of the objects 5 earth and moon are kept in there respective orbits due to the influence of a inertia b gravity c gravity and inertia* d neither gravity or inertia if you answer all questions right i will give
Answers: 1
You know the right answer?
5.96 g of ammonia reacts completely according to the following reaction:
2 NH3, (g) + Co2, (g) → CN...
Questions in other subjects:

Biology, 26.01.2021 23:00

Mathematics, 26.01.2021 23:00

Mathematics, 26.01.2021 23:00


Mathematics, 26.01.2021 23:00

Mathematics, 26.01.2021 23:00

Mathematics, 26.01.2021 23:00


Mathematics, 26.01.2021 23:00

History, 26.01.2021 23:00