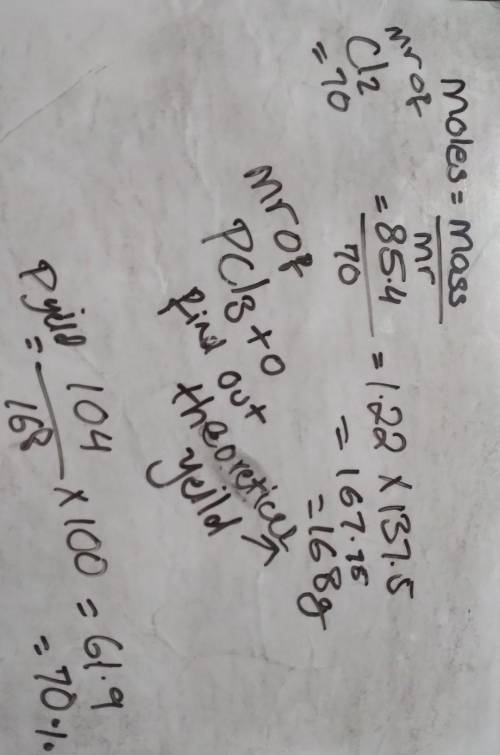Chemistry, 05.06.2021 22:40 Angeldelissa
85.4 g of chlorine reacts completely according to the following reaction: P4 (s) + 6 Cl2, (g) 4PC13 (I)
If 104 g of phosphorous trichloride is produced, what is the percent yield for this reaction?
please show work
will give brainliest

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:50, leannamat2106
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1
You know the right answer?
85.4 g of chlorine reacts completely according to the following reaction: P4 (s) + 6 Cl2, (g) 4PC13...
Questions in other subjects:







Mathematics, 24.12.2019 07:31


Advanced Placement (AP), 24.12.2019 08:31

Mathematics, 24.12.2019 08:31