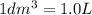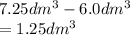Chemistry, 04.06.2021 20:20 juliannabartra
Propane burns to form carbon dioxide and water. The equation for the reaction is:
C 3 H 8 (g)+5 O 2 (g) 3 CO 2 (g)+4 H 2 O(l)
3.60d * m ^ 3 carbon dioxide is produced when a sample of propane is burned in 7.25d * m ^ 3 oxygen.
Calculate the volume of unreacted oxygen. Give your answer in cm^ 3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

You know the right answer?
Propane burns to form carbon dioxide and water. The equation for the reaction is:
C 3 H 8 (g)+5 O 2...
Questions in other subjects:

History, 20.04.2020 03:30



History, 20.04.2020 03:31




Mathematics, 20.04.2020 03:31

Chemistry, 20.04.2020 03:31