
Chemistry, 04.06.2021 18:00 gissellesolorza
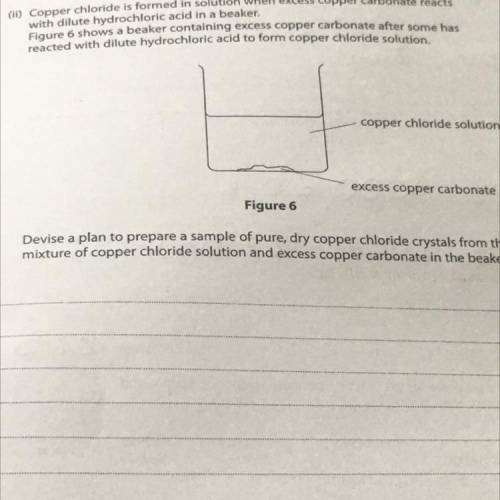
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochloric acid in a beaker.
Figure 6 shows a beaker containing excess copper carbonate after some has
reacted with dilute hydrochloric acid to form copper chloride solution.
DO NOT WRITE IN THIS AREA
DO NOT WRITE IN THIS AREN
copper chloride solution
excess copper carbonate
Figure 6
Devise a plan to prepare a sample of pure, dry copper chloride crystals from the
mixture of copper chloride solution and excess copper carbonate in the beaker.
DO NOT
THIS AREA
A

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2


Chemistry, 22.06.2019 04:30, mamabates181981
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1
You know the right answer?
(11) Copper chloride is formed in solution when excess copper carbonate reacts
with dilute hydrochl...
Questions in other subjects:



Mathematics, 05.04.2020 22:20






History, 05.04.2020 22:21



