
Chemistry, 04.06.2021 06:50 donnafranks2003
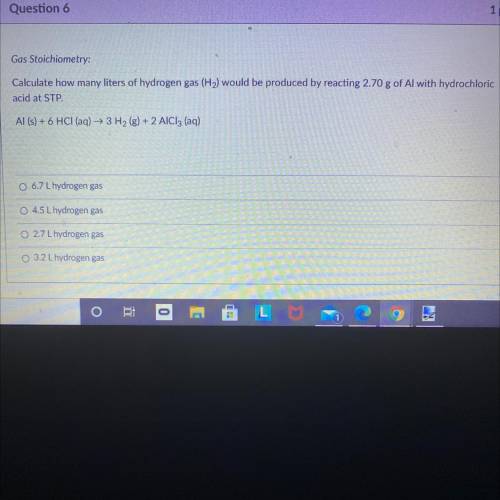
Gas Stoichiometry:
Calculate how many liters of hydrogen gas (H2) would be produced by reacting 2.70 g of Al with hydrochloric
acid at STP.
Al (s) + 6 HCl (aq) + 3H2(g) + 2 AlCl3 (aq)
6.7 L hydrogen gas
O 4.5 L hydrogen gas
O 2.7 L hydrogen gas
3.2 L hydrogen gas

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:20, banna01man
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1

Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Gas Stoichiometry:
Calculate how many liters of hydrogen gas (H2) would be produced by reacting 2.7...
Questions in other subjects:


English, 05.10.2020 15:01

Mathematics, 05.10.2020 15:01


Chemistry, 05.10.2020 15:01

Chemistry, 05.10.2020 15:01

Business, 05.10.2020 15:01



Mathematics, 05.10.2020 15:01



