Chemistry, 04.06.2021 04:30 krazyapril4601
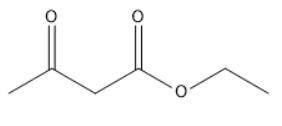
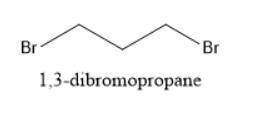
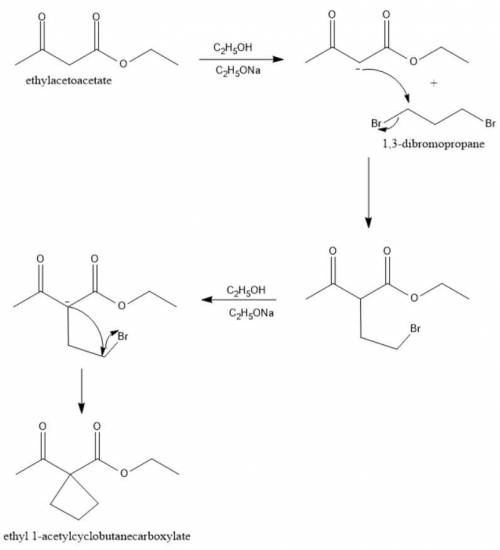
When Ethyl Acetoacetate is treated with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol, a compound is produced that has the formula C9H14O3. This compound has an infrared spectrum that shows only one carbonyl adsorption and no OH bond stretch. Suggest a structure for this compound, and provide a mechanism for its formation.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1


Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
When Ethyl Acetoacetate is treated with 1,3-Dibromopropane and 2 moles of Sodium Ethoxide in Ethanol...
Questions in other subjects:







Mathematics, 25.02.2021 17:50