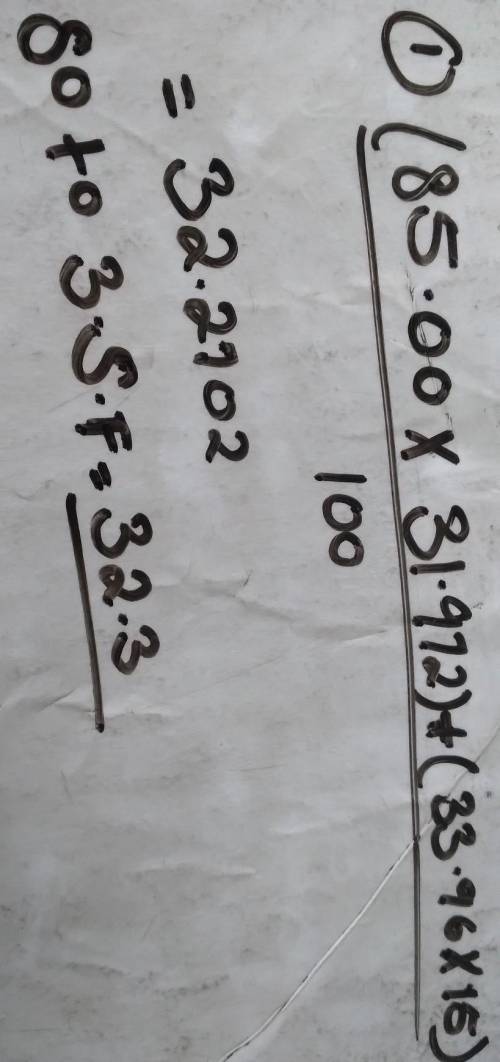Chemistry, 03.06.2021 21:20 birdman37361
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 amu (85.00 % abundance) and 33.96 amu (15.00% abundance)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:00, kaliyab191
Diamond, graphite, and fullerenes share what property? a. they are all made of carbon (c) bonded to a metal. b. their shape. c. they are all made of carbon (c). d. they are all good conductors.
Answers: 1


You know the right answer?
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 a...
Questions in other subjects:



Business, 07.01.2020 21:31

Mathematics, 07.01.2020 21:31





Biology, 07.01.2020 21:31

Social Studies, 07.01.2020 21:31