

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Write balanced equations and solubility product expressions for the following compounds
a. CuBr
Questions in other subjects:

Chemistry, 29.04.2021 21:20



Mathematics, 29.04.2021 21:20


Mathematics, 29.04.2021 21:20

English, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20

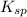 .
.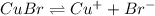
![K_{sp}=[Cu^+][Br^-]](/tpl/images/1361/6455/12867.png)
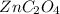
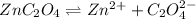
![K_{sp}=[Zn^{2+}][C_2O_4^{2-}]](/tpl/images/1361/6455/9c1eb.png)
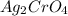
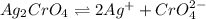
![K_{sp}=[Ag^{+}]^2[CrO_4^{2-}]](/tpl/images/1361/6455/72845.png)
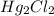 into its ions follows:
into its ions follows: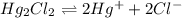
![K_{sp}=[Hg^{+}]^2[Cl^{-}]^2](/tpl/images/1361/6455/c5176.png)
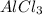
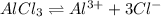
![K_{sp}=[Al^{3+}][Cl^{-}]^3](/tpl/images/1361/6455/2ad29.png)
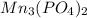 into its ions follows:
into its ions follows: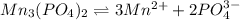
![K_{sp}=[Mn^{2+}]^3[PO_4^{3-}]^2](/tpl/images/1361/6455/a7ec5.png)


