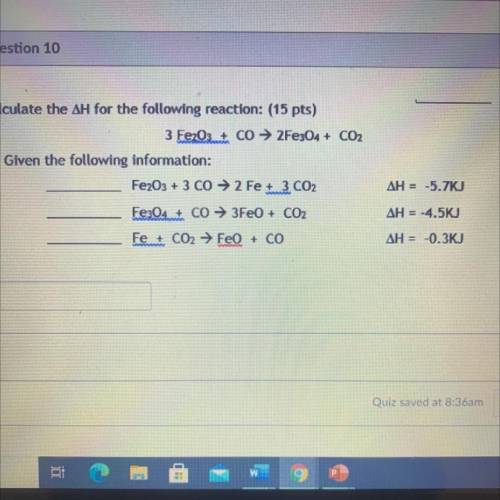
Calculate the ΔH for the following reaction: (15 pts)
3 Fe2O3+ Co → 2Fe304 + CO2
Given the fo...

Chemistry, 03.06.2021 17:00 hjeffrey168
Calculate the ΔH for the following reaction: (15 pts)
3 Fe2O3+ Co → 2Fe304 + CO2
Given the following information:
Fe2O3 + 3 CO → 2 Fe 3 CO2
ΔH = -5.7KJ
Fe3O4 + CO → 3FeO + CO2
ΔH = -4.5KJ
Fe + CO2 → FeO + CO
ΔH = -0.3KJ

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, nadiarose6345
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2


Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
Questions in other subjects:

Biology, 20.07.2019 05:00





English, 20.07.2019 05:00

English, 20.07.2019 05:00

English, 20.07.2019 05:00


English, 20.07.2019 05:00



