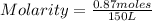Chemistry, 03.06.2021 02:20 emfastback8868
40.0g of ethanol, C2H5OH, is dissolved in 150.0L of solution. Determine the molarity of ethanol in this solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
40.0g of ethanol, C2H5OH, is dissolved in 150.0L of solution. Determine the molarity of ethanol in t...
Questions in other subjects:

Mathematics, 04.01.2020 09:31


Geography, 04.01.2020 09:31


Mathematics, 04.01.2020 09:31


Mathematics, 04.01.2020 09:31



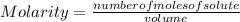
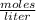 .
.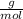 , then the number of moles that 40 grams of ethanol contain is calculated by:
, then the number of moles that 40 grams of ethanol contain is calculated by: