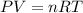Chemistry, 03.06.2021 01:00 piratesfc02
What volume of hydrogen will produced at 1.45 atm and a tempeture of 20C by the reaction of 37.6g of magnesium 1Mg+2H2O--> Mg(OH)2+H2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
What volume of hydrogen will produced at 1.45 atm and a tempeture of 20C by the reaction of 37.6g of...
Questions in other subjects:

History, 13.04.2021 18:30

Biology, 13.04.2021 18:30




Mathematics, 13.04.2021 18:30


Mathematics, 13.04.2021 18:30