Identify the type of chemical reaction below:
CaCO3(s) CaO(s) + CO2(g)
...

Chemistry, 02.06.2021 22:00 ejohnstonee111
Identify the type of chemical reaction below:
CaCO3(s) CaO(s) + CO2(g)
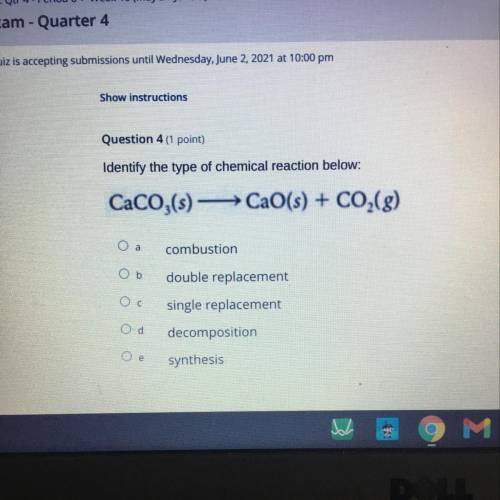

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2


Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 26.01.2020 07:31




Physics, 26.01.2020 07:31





Physics, 26.01.2020 07:31