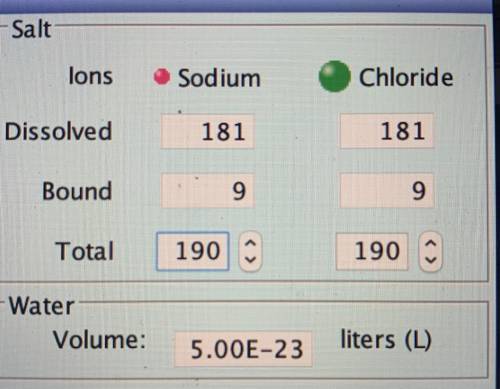
The solubility of an ionic compound can be expressed as the number of moles of the compound that will dissolve per liter of solution (molarity). The saturated solution has approximately(a) sodium ions dissolved in it (give an estimate of the average value.) The solution (not the solid) contains approximately(b) moles of sodium ions.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, dondre54
Use the image below to answer the following questions there are three types of heat transfer occurring in the picture below. write a sentence about where each is occurring. (total three complete sentences in your own words.) on a very cold day, if you place your hand towards the bottom of a window, you can feel the convection of cold air as the cold, dense air sinks. try it out. did you feel the convection? it's a marshmallow on a metal stick over fire
Answers: 3

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
The solubility of an ionic compound can be expressed as the number of moles of the compound that wil...
Questions in other subjects:

Mathematics, 17.10.2020 07:01



Social Studies, 17.10.2020 07:01




Social Studies, 17.10.2020 07:01


History, 17.10.2020 07:01