
Chemistry, 01.06.2021 20:40 annapittbull12
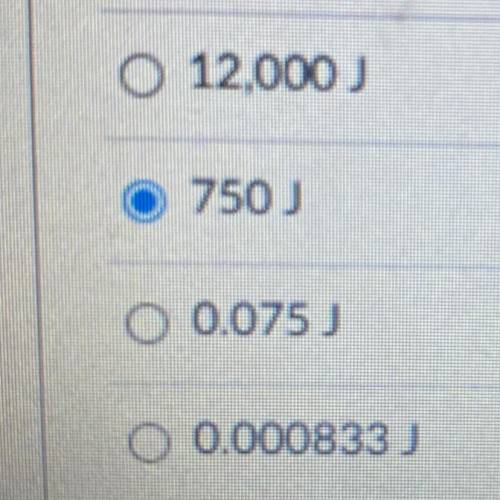
A 100. gram sample of a metal undergoes a temperature change from 20.°C to 50.°C after absorbing heat. How much heat was absorbed?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, genyjoannerubiera
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1



Chemistry, 23.06.2019 08:50, leah5981
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas. caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible. relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
A 100. gram sample of a metal undergoes a temperature change from 20.°C to 50.°C after
absorbing he...
Questions in other subjects:




Social Studies, 07.10.2021 23:50

Mathematics, 07.10.2021 23:50

Mathematics, 07.10.2021 23:50




Computers and Technology, 07.10.2021 23:50



