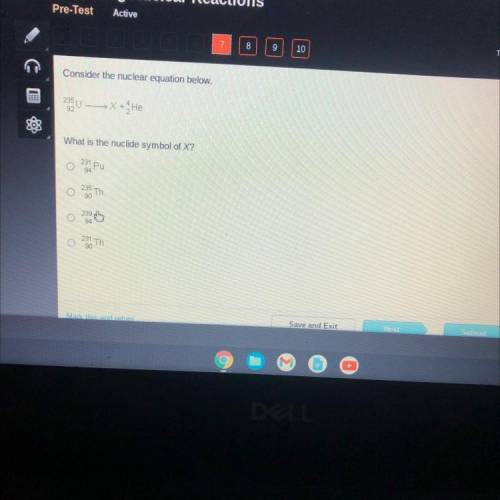
Consider the nuclear equation below.
SESE
235 U
X + He
92
What is the nucli...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, shafferakr6
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3


Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 27.01.2021 18:00


Mathematics, 27.01.2021 18:00


Mathematics, 27.01.2021 18:00


Mathematics, 27.01.2021 18:00

Social Studies, 27.01.2021 18:00

Mathematics, 27.01.2021 18:00