
Chemistry, 01.06.2021 19:00 okokalyssa
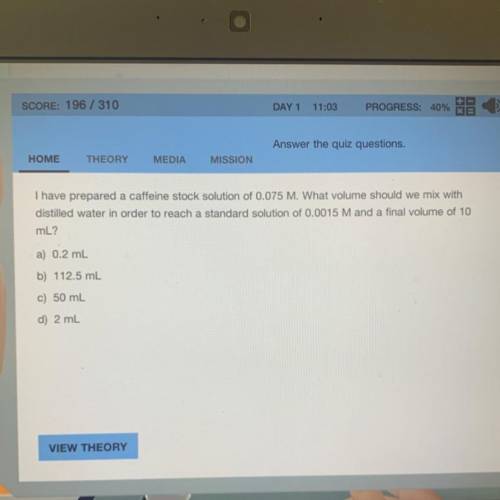
I have prepared a caffeine stock solution of 0.075 M. What volume should we mix with
distilled water in order to reach a standard solution of 0.0015 M and a final volume of 10
mL?
a) 0.2 mL
b) 112.5 mL
c) 50 mL
d) 2 mL
PLEASE HELP!
No Links!
I will award brainliest to the first person to answer

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 16:40, westball101
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
I have prepared a caffeine stock solution of 0.075 M. What volume should we mix with
distilled wate...
Questions in other subjects:

Mathematics, 13.01.2021 20:10


Mathematics, 13.01.2021 20:10


Mathematics, 13.01.2021 20:10



Mathematics, 13.01.2021 20:10

History, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10



