
Chemistry, 01.06.2021 16:10 aseel667789
An investigation is conducted into how the mass of magnesium metal reacting with hydrochloric acid affects the amount of hydrogen gas produced.
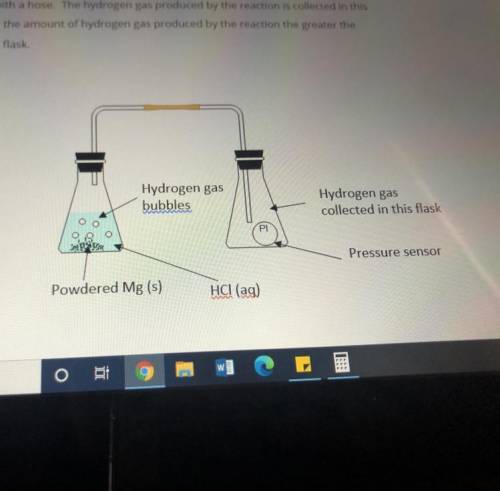
Masses of 0.10g, 0.20g, 0.30g and 0.40g of powdered Mg metal are reacted with hydrochloric acid(HCl). The conical flask containing the reaction mixture of Mg and HCl is connected to another conical flask with a hose. The hydrogen gas produced by the reaction is collected in this conical flask. The greater the amount of hydrogen gas produced by the reaction the greater the pressure of the gas in the flask.
A) what is the independent variable:
B) what is the dependent variable:
C) write a hypothesis for this investigation:
D) give 2 variables that should have been controlled for this investigation:

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 06:40, CylieTbh
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 10:30, kylemartinez13
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2
You know the right answer?
An investigation is conducted into how the mass of magnesium metal reacting with hydrochloric acid a...
Questions in other subjects:

Spanish, 25.05.2021 17:10






History, 25.05.2021 17:10






