
Chemistry, 01.06.2021 14:00 erickcastillo9124
a buffer solution contain 0.1 mole per litres of acetic acid and 0.001 moles perlitre of sodium acetate. what will be its pH?(k=1.8×10-5)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

You know the right answer?
a buffer solution contain 0.1 mole per litres of acetic acid and 0.001 moles perlitre of sodium acet...
Questions in other subjects:

Biology, 05.07.2019 10:00






English, 05.07.2019 10:00

Geography, 05.07.2019 10:00

Mathematics, 05.07.2019 10:00

![pH=pK_a+\log (\frac{\text{[conjugate base]}}{\text{[acid]}})](/tpl/images/1357/3102/8e22a.png) .......(1)
.......(1) ......(2)
......(2)

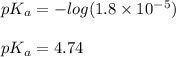
 = 0.1 M
= 0.1 M = 0.001 M
= 0.001 M


