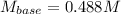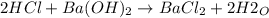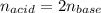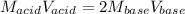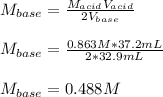Chemistry, 31.05.2021 04:40 aliciaa101
A 32.9-mL sample of Ba(OH)2 is titrated with HCl. If 37.2 mL of 0.863 M HCl is needed to reach the endpoint, what is the concentration (M) of the Ba(OH)2 solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, daryondaniels28
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 05:50, ttangelique
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
A 32.9-mL sample of Ba(OH)2 is titrated with HCl. If 37.2 mL of 0.863 M HCl is needed to reach the e...
Questions in other subjects:


Health, 08.12.2020 05:50

Mathematics, 08.12.2020 05:50


Mathematics, 08.12.2020 05:50

History, 08.12.2020 05:50