

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, coolkid2041
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Given the following reaction:
2 ZnS(s) + 3 O2(g) → 2 ZnO(s) + 2 SO2(g) ΔH = -927.54 kJ
...
...
Questions in other subjects:


Physics, 06.06.2020 01:01

History, 06.06.2020 01:01







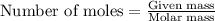 ......(1)
......(1)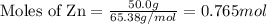
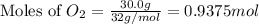

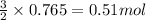 of oxygen gas
of oxygen gas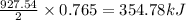 of energy
of energy

