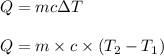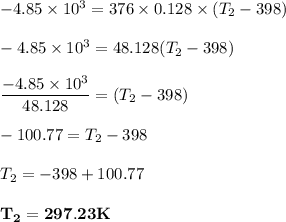Chemistry, 29.05.2021 02:50 awesomegrill
determine the final temperature of a gold nugget parentheses Mass equals 376 G parentheses that starts at 398k and loses 4.85 KJ of heat to a snowbank when it is lost. The specific heat capacity of gold is 0.128 J/g degrees C

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 12:30, ella3714
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1

Chemistry, 23.06.2019 12:40, valleriieZ7002
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2

Chemistry, 23.06.2019 16:40, raincalderxn
Which statement is true about market economies? government goals drive business decisions. people have the freedom to choose their jobs. several are market economies vist around the world
Answers: 2
You know the right answer?
determine the final temperature of a gold nugget parentheses Mass equals 376 G parentheses that star...
Questions in other subjects:


Arts, 15.11.2020 22:40

Mathematics, 15.11.2020 22:40



History, 15.11.2020 22:40


Mathematics, 15.11.2020 22:40

Computers and Technology, 15.11.2020 22:40

English, 15.11.2020 22:40

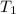 = 398 K
= 398 K