
Chemistry, 28.05.2021 20:00 pedropaulofpedrosapp
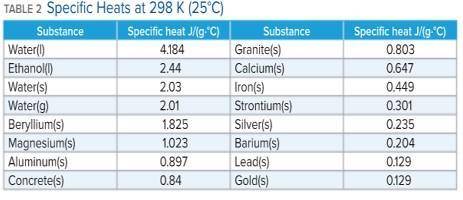
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substance absorbed 2440 J of energy. What is the specific heat of the substance? Identify the substance among those listed in Table 2.
A. the specific heat is 0.897 J/g. C, The Substance is aluminum
B. the specific heat is -0.897 J/g. C, The Substance is aluminum
C. the specific heat is 4.184 J/g. C, The Substance is water
D. there's not enough information to determine which is the substance.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, micahwilkerson9495
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 17:30, llamasking
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 19:40, jholland03
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substa...
Questions in other subjects:




Mathematics, 10.02.2020 09:05

Mathematics, 10.02.2020 09:05

Mathematics, 10.02.2020 09:05


English, 10.02.2020 09:06


Mathematics, 10.02.2020 09:06



