
Chemistry, 28.05.2021 04:20 brillamontijo
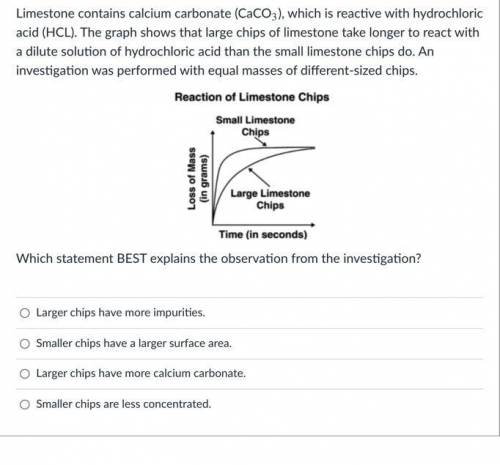
Limestone contains calcium carbonate (CaCO3), which is reactive with hydrochloric acid (HCL). The graph shows that large chips of limestone take longer to react with a dilute solution of hydrochloric acid than the small limestone chips do. An investigation was performed with equal masses of different-sized chips.
Which statement BEST explains the observation from the investigation?
Group of answer choices
Larger chips have more impurities.
Smaller chips have a larger surface area.
Larger chips have more calcium carbonate.
Smaller chips are less concentrated.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 18:00, brisacruz013
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
Limestone contains calcium carbonate (CaCO3), which is reactive with hydrochloric acid (HCL). The gr...
Questions in other subjects:










Arts, 16.03.2020 18:56



