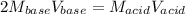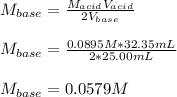Chemistry, 28.05.2021 01:10 dbzrules02
It takes 32.35 mL of a 0.0895 M hydrochloric acid solution to reach the equivalence point in the reaction with 25.00 mL of barium hydroxide. 2HCl(aq) Ba(OH)2(aq) 2H2O(l) BaCl2(aq) What is the molar concentration of the barium hydroxide solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
It takes 32.35 mL of a 0.0895 M hydrochloric acid solution to reach the equivalence point in the rea...
Questions in other subjects: