Chemistry, 27.05.2021 22:50 rachellynn02
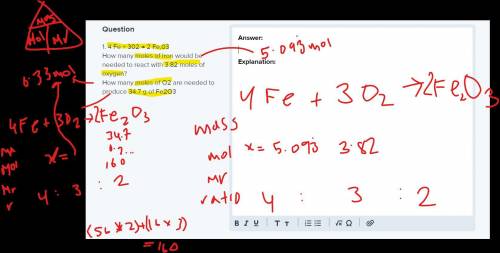
1. 4 Fe + 302 → 2 Fe,03
How many moles of iron would be needed to react with 3.82 moles of oxygen?
How many moles of O2 are needed to produce 34.7 g of Fe2O3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 08:00, celestemaria0727
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 12:00, angtrevv
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
1. 4 Fe + 302 → 2 Fe,03
How many moles of iron would be needed to react with 3.82 moles of oxygen?<...
Questions in other subjects:



Biology, 21.07.2019 16:00



Mathematics, 21.07.2019 16:00