
Chemistry, 27.05.2021 21:30 moneybabyy38
One of the main components of an airbag is the gas that fills it. As part of the design process, you need to determine the exact amount of nitrogen that should be produced. Calculate the number of moles of nitrogen required to fill the airbag. Show your work. Assume that the nitrogen produced by the chemical reaction is at a temperature of 495°C and that nitrogen gas behaves like an ideal gas.

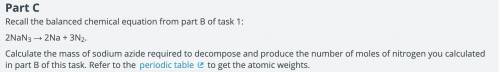

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pollywallythecat
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 03:50, Pizzapegasus1
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
One of the main components of an airbag is the gas that fills it. As part of the design process, you...
Questions in other subjects:


Biology, 16.12.2020 20:00

Biology, 16.12.2020 20:00

History, 16.12.2020 20:00


English, 16.12.2020 20:00


Mathematics, 16.12.2020 20:00


Geography, 16.12.2020 20:00



