
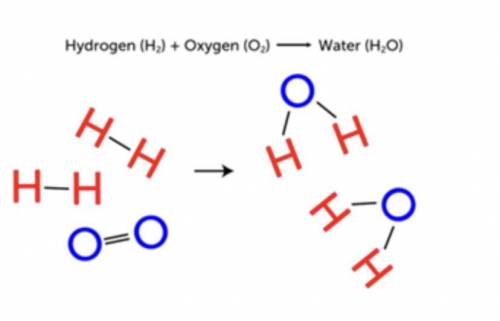
In Figure on the right, you see how hydrogen and oxygen combine chemically to form water.
a. How could you use chemical symbols and formulas to represent this reaction?
b. How many molecules of hydrogen and oxygen are involved in this reaction?
c. How many molecules of water are produced? How could you include these numbers in your representation of the reaction?
d. Which bonds were broken and which bonds were formed in this reaction?

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
In Figure on the right, you see how hydrogen and oxygen combine chemically to form water.
a. How co...
Questions in other subjects:








Mathematics, 06.04.2021 17:20





