
The shaded regions X and Y indicate changes in the energy level of the system. Which of the following correctly identifies these shaded areas?
A. Region X = energy released by the system at the start, Region Y = energy released by the system at the end
B. Region X = energy difference between reactants and products, Region Y = sum of energy of reactants and products
C. Region X = energy required to initiate the reaction, Region Y = energy released by the reaction
D. Region X = energy gained by the surroundings of the system, Region Y = energy lost by the surroundings of the system
There is a screen shot
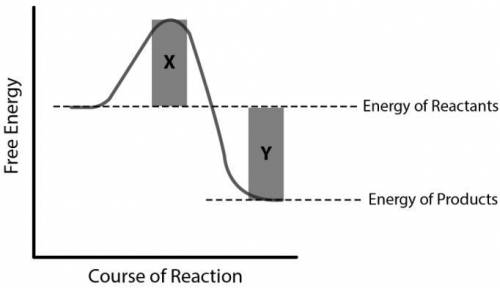

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:10, Jana1517
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes. which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature. sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1

Chemistry, 22.06.2019 20:10, maddie1776
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1


Chemistry, 23.06.2019 00:30, terryg4397
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
The shaded regions X and Y indicate changes in the energy level of the system. Which of the followin...
Questions in other subjects:

Mathematics, 25.04.2021 07:50




Mathematics, 25.04.2021 07:50

Mathematics, 25.04.2021 07:50



Mathematics, 25.04.2021 07:50

Mathematics, 25.04.2021 07:50



