Chemistry, 27.05.2021 09:30 nikeahbrown
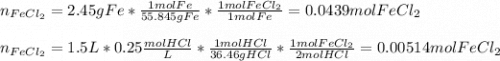
If 2.45 g of iron are placed in 1,5 L of 0.25M HCl, how many grams of FeCl2 are obtained? Identify the limiting and excess reactants in this single replacement reaction. Fe + 2HCl = FeCl2 + H2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, aidengalvin20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
If 2.45 g of iron are placed in 1,5 L of 0.25M HCl, how many grams of FeCl2 are obtained? Identify t...
Questions in other subjects:


English, 17.03.2020 18:42





Biology, 17.03.2020 18:42



Chemistry, 17.03.2020 18:43