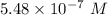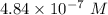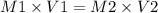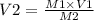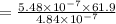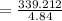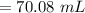A chemist must dilute 61.9 mL of 548. nM aqueous sodium carbonate (Na2CO3) solution until the concentration falls to 484. nM . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, itsyaboiamo
Why should the scientific method be used to answer a question? a. it provides a way to test an idea without any bias. b. it provides a way to test a hypothesis. c. it provides a way to ensure all hypotheses are proven correct. d. it provides a way to quickly turn a hypothesis into a scientific theory.
Answers: 1

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


You know the right answer?
A chemist must dilute 61.9 mL of 548. nM aqueous sodium carbonate (Na2CO3) solution until the concen...
Questions in other subjects:

English, 02.10.2021 14:00

Business, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00





Mathematics, 02.10.2021 14:00