Chemistry, 26.05.2021 21:10 savannahvargas512
PLZ Help asap
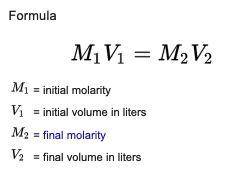
Sulfuric acid is purchased full-strength at 18.0M. How many milliliters of full-strength acid are needed to prepare 500.0 mL of aqueous 0.35M H2SO4? Round your answer to 3 significant figures.
A. 13.4 mL
B. 82.6 mL
C. 9.70 mL
D. 79.0 mL

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, kakesheco4210
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 04:20, lindseysmith9522
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
PLZ Help asap
Sulfuric acid is purchased full-strength at 18.0M. How many milliliters of full-stren...
Questions in other subjects:

Mathematics, 25.10.2019 23:43



Mathematics, 25.10.2019 23:43

Mathematics, 25.10.2019 23:43




Mathematics, 25.10.2019 23:43