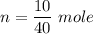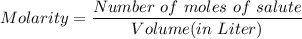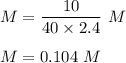Chemistry, 26.05.2021 20:30 hamilclips6805
What is the molarity of a solution prepared by dissolving 10.0 grams of NaOH in enough water to make a solution with a total volume of 2.40 liters?
O0.104 M NaOH
O0.201 M NaOH
O0.361 M NaOH
O0.412 M NaOH

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, arnold2619
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 21.06.2019 17:30, hannahkelly3618
How many moles are equivalent to 55.5g of nano3
Answers: 1

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 23:00, autumperry3599
What is the chemical formula for dihydrogen monoxide
Answers: 2
You know the right answer?
What is the molarity of a solution prepared by dissolving 10.0 grams of NaOH in enough water to make...
Questions in other subjects:

Mathematics, 20.02.2021 18:40




Physics, 20.02.2021 18:40




English, 20.02.2021 18:40

Geography, 20.02.2021 18:40