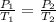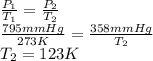Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Calculate the new temperature when a container of
gas has a pressure of 795 mm Hg at 273 K and the<...
Questions in other subjects:


Mathematics, 28.12.2020 08:30



English, 28.12.2020 08:30



Mathematics, 28.12.2020 08:30


World Languages, 28.12.2020 08:30

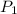 = 795 mm Hg,
= 795 mm Hg, 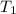 = 273 K
= 273 K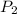 = 358 mm Hg,
= 358 mm Hg, 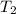 = ?
= ?