Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
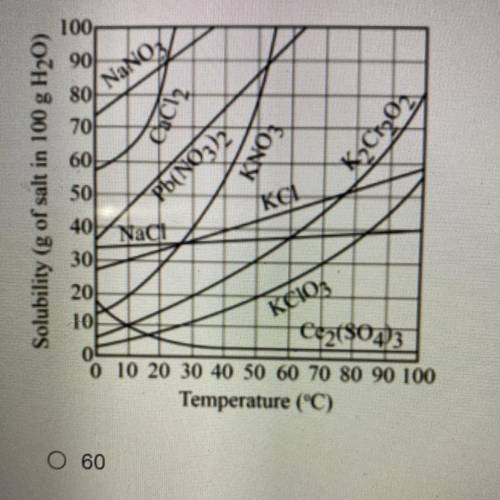
Refer to the graph below. In order to have a saturated solution, how many grams of potassium nitrate...
Questions in other subjects:

History, 11.05.2021 09:50

English, 11.05.2021 09:50




English, 11.05.2021 09:50