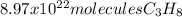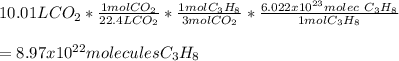Chemistry, 26.05.2021 06:20 darkremnant14
Propane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water.
C3H3 +502 – 3 CO2 + 4H20
Determine the number of molecules of propane needed to produce 10.01 liters of carbon dioxide

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 15:00, cynthiagutierrez65
Where can i find naap lab answers sheet/key?
Answers: 1

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Propane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water....
Questions in other subjects:

Mathematics, 13.07.2019 04:00

Mathematics, 13.07.2019 04:00

English, 13.07.2019 04:00

Mathematics, 13.07.2019 04:00



Social Studies, 13.07.2019 04:00