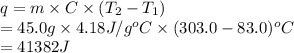Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:40, wbrandi118
Water ionizes by the equation h2o(l)⇌h+(aq)+oh−(aq) the extent of the reaction is small in pure water and dilute aqueous solutions. this reaction creates the following relationship between [h+] and [oh−]: kw=[h+][oh−] keep in mind that, like all equilibrium constants, the value of kw changes with temperature.
Answers: 1

Chemistry, 22.06.2019 04:00, nikkih1225
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
How much energy must be absorbed by 45.0g of water to increase its temperature from 83 0°C to 303.0°...
Questions in other subjects:



Geography, 17.10.2019 02:30

Mathematics, 17.10.2019 02:50


Chemistry, 17.10.2019 02:50

Mathematics, 17.10.2019 02:50

Mathematics, 17.10.2019 02:50


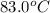 to
to 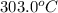 .
.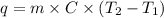
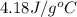 (here, for water)
(here, for water)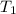 = initial temperature
= initial temperature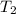 = final temperature
= final temperature