Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters...

Chemistry, 25.05.2021 19:20 issacbeecherpebpyl
Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters of O2 (g)
would be required to produce 20 liters of NO (g)?
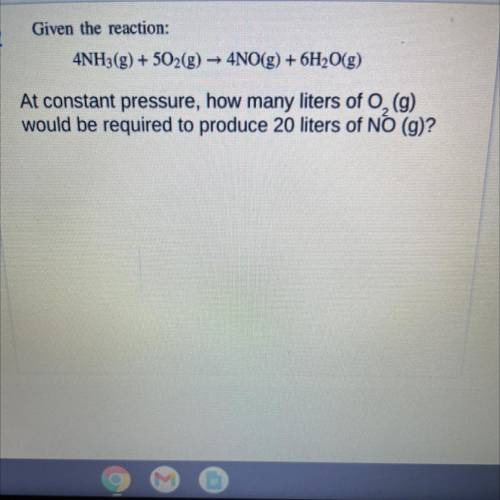

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 22.06.2019 22:00, Porciabeauty6788
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 19.05.2020 02:06



Mathematics, 19.05.2020 02:06


Mathematics, 19.05.2020 02:06


Mathematics, 19.05.2020 02:06

History, 19.05.2020 02:06




