
Chemistry, 25.05.2021 17:20 jessie8022
What is the molarity of a solution made from dissolving 3.0 moles of KCl in 500 mL of Solution?
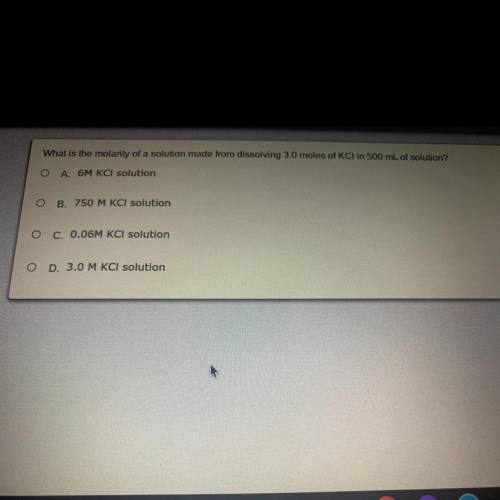

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, MrSavannahCat
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 08:20, pilarmonsivais
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3


Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
What is the molarity of a solution made from dissolving 3.0 moles of KCl in 500 mL of Solution?
Questions in other subjects:

Mathematics, 05.05.2021 21:20

Chemistry, 05.05.2021 21:20



Health, 05.05.2021 21:20

History, 05.05.2021 21:20

Chemistry, 05.05.2021 21:20





