
Chemistry, 25.05.2021 08:20 Christiancameron1234
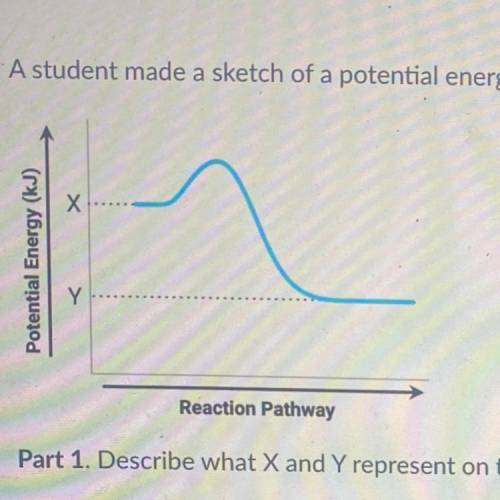
A student made a sketch of a potential energy diagram to represent an exothermic reaction.
Potential Energy (kJ)
Y
Reaction Pathway
Part 1. Describe what X and Y represent on the diagram.
Part 2. Explain how to determine the enthalpy change of a reaction on the diagram.
Part 3. Explain why the diagram made by the student is correct or incorrect.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2

Chemistry, 23.06.2019 00:00, kittenalexis68
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
A student made a sketch of a potential energy diagram to represent an exothermic reaction.
Potentia...
Questions in other subjects:

History, 06.07.2019 09:00

Chemistry, 06.07.2019 09:00


Chemistry, 06.07.2019 09:00


Social Studies, 06.07.2019 09:00

Mathematics, 06.07.2019 09:00

Advanced Placement (AP), 06.07.2019 09:00




