Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
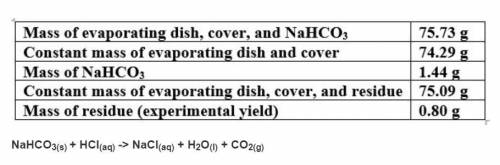
Based on a theoretical yield of 1.00 grams NaCl, calculate the mass in grams of hydrochloric acid yo...
Questions in other subjects:



Business, 27.02.2020 04:29


Mathematics, 27.02.2020 04:29


Mathematics, 27.02.2020 04:29



History, 27.02.2020 04:29