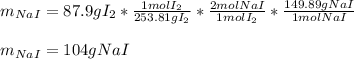Chemistry, 24.05.2021 17:30 Kenzie5755
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution containing sodium iodide. 2NaI(aq)+Cl2(g)⟶I2(s)+2NaCl(aq) How many grams of sodium iodide, NaI, must be used to produce 87.9 g of iodine, I2?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 07:30, isalih7256
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution c...
Questions in other subjects:


History, 13.10.2021 19:50

Biology, 13.10.2021 19:50

Mathematics, 13.10.2021 19:50

Chemistry, 13.10.2021 20:00




Mathematics, 13.10.2021 20:10

Mathematics, 13.10.2021 20:10