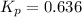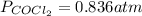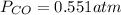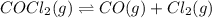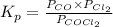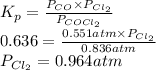The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g) + Cl2(g)
If an equilibrium mixture of the three gases in a 16.9 L container at 600K contains COCl2 at a pressure of 0.836 atm and CO at a pressure of 0.551 atm, the equilibrium partial pressure of Cl2 is atm.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1
You know the right answer?
The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g)...
Questions in other subjects:



Computers and Technology, 22.06.2019 14:00


Biology, 22.06.2019 14:00


Business, 22.06.2019 14:00

Mathematics, 22.06.2019 14:00


Spanish, 22.06.2019 14:00

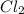 is 0.964 atm.
is 0.964 atm.