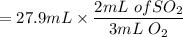Chemistry, 24.05.2021 09:20 Destinationz
Calculate the volume of sulfur dioxide produced when 27.9 mL O2 reacts with carbon disulfide.
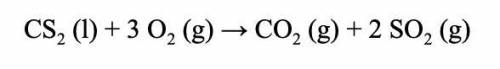

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Calculate the volume of sulfur dioxide produced when 27.9 mL O2 reacts with carbon disulfide.
...
...
Questions in other subjects:


Mathematics, 23.10.2020 18:00

Mathematics, 23.10.2020 18:00



Chemistry, 23.10.2020 18:00

History, 23.10.2020 18:00