Chemistry, 24.05.2021 09:10 alexkrol30083
A 5.00 mL sample of hydrochloric acid is titrated with 0.1293 M ammonia (a base). If the titration required 28.15 mL of ammonia, determine the following:
the original concentration of the acid
the original pH of the acid

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 23.06.2019 14:30, ajahbraun
Recognizing the properties of water water has a "bent" geometry. which explanation does not explain why? o water's oxygen has unbonded electron pairs that repel each other. water can form hydrogen bonds. electrons are evenly distributed in the water molecule. do ne
Answers: 3

Chemistry, 23.06.2019 15:30, georgesarkes12
Floor 19 is an isotope of fluorine it has different number a. electrons b. protons c. neutrons d. electron shells
Answers: 1

Chemistry, 23.06.2019 18:00, keshewar4427
Amolecule is a(n) - a. element that physically combines with another element. b. particle composed of two or more atoms bonded together covalently. c. element that isn't bonded to another element.
Answers: 1
You know the right answer?
A 5.00 mL sample of hydrochloric acid is titrated with 0.1293 M ammonia (a base). If the titration r...
Questions in other subjects:





Mathematics, 07.01.2021 18:40

Arts, 07.01.2021 18:40

Social Studies, 07.01.2021 18:40



Chemistry, 07.01.2021 18:40

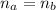

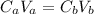
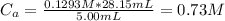
![pH = -log([H^{+}])](/tpl/images/1343/3808/0d4b9.png)