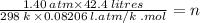Chemistry, 23.05.2021 14:00 ian2006huang
A sample of neon gas at 25.0°C has a volume of 42.4 L and a pressure of 1.40 atm. How many moles of neon are there? NOTE: You must show your calculation on the attached scratch paper,
including which of the Gas Law formulas you used. *
A. 14.4 moles
B. 0.41 moles
C. 28.9 moles
D. 2.43 moles
(Show how you did it please)


Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

You know the right answer?
A sample of neon gas at 25.0°C has a volume of 42.4 L and a pressure of 1.40 atm. How many moles of...
Questions in other subjects:


Mathematics, 21.05.2021 17:20



Computers and Technology, 21.05.2021 17:20

English, 21.05.2021 17:20